With the passage of the 1990 Clean Air Act Amendments, many chemical and metal industries and utility producers will now be required to further limit the amount of NOx (Nitrogen Oxides) produced. NOx is a precursor to ozone in the atmosphere, and is believed to be a major contributor to acidic deposition (acid rain).
Formation of ozone:

NOx is produced in a variety of different processes, including combustion equipment, gas turbines, incinerators, kilns and power plants. NOx also is emitted as by product from many metal treatment processes where nitric acid is used as an oxidant. Plating or catalyst recovery involves the reaction of nitric acid and transition metals also forming NOx. Substantial amounts of NOx also can be generated in the specialty chemical industry when nitric acid is used as a reagent.
The denitration processes for removal of NOx are classified into two groups: in one, NOx is absorbed by means of solutions, and in the other NOx is reduced to N2 by means of a reducing gas under the presence of a catalyst.
Selective catalytic reduction (SCR) is a chemical process that changes the oxides of nitrogen into N2 and H2O. The reactions take place at a temperature of 600-750 F in the presence of a catalyst. Ammonia is injected into the exhaust gases prior to their passing into the SCR. NOx removal efficiencies with SCR range from 80 to 90% [2]. An NH3/NOx mole ratio of 1.0 to 1.5 is normally used although the theoretical ratio is about 0.67. Although a portion of the excess ammonia decomposes in the reactor, a considerable amount of it would remain in the treated gas and may cause problems. For example, ammonia may combine with SO3, which is present in a small amount even after the wet scrubbing, to form ammonium bisulfate which condenses in a heat exchanger. However, catalysts are affected by dust and most are poisoned by sulfur and chlorine compounds.
For treatment of contaminated gas, a wet removal process may be carried out first to remove dust and poisonous chemicals, but in this case, the gas temperature drops to 120-150 and must then be reheated to 600-750 F, a large heat exchanger and a considerable amount of fuel are needed. In addition, mists from the scrubber may cause corrosion of the heat exchanger and contamination of the catalyst. Over a period of time, the materials in the catalyst-ceramics and zeolites degrade and must be replaced depending upon the industrial sources of the gases. Because the catalysts are made up of heavy metals, disposal of spent catalysts can also be a problem.
In recent years, many wet processes for NOx removal have been developed with the aim of removing NOx and SOx simultaneously. Scrubbing process also used to remove NOx from NOx rich gases is produced in a relatively small amount at metal-dissolving, nitric acid and chemical plants, etc.
This article describes the NOx chemical reaction associated with wet removal processes. The aim, then, is to provide a reasonably clear and uncomplicated basis for evaluation of potential treatment methods to help determine which may be practically applicable in a given situation.
Several oxides of nitrogen are found in the atmosphere but only nitric oxide (NO) and nitrogen dioxide (NO2) are important as air pollutants. The symbol NOx is frequently used to represent the composite of the two. The other nitrogen oxides seldom occur in appreciable quantities and then only under special conditions.
Essentially, NOx contains nitric oxide and nitrogen dioxide in varying proportions. This fluctuating ratio, and the fact that these compounds exhibit quite different properties when contacted with water (as would occur in a wet scrubber) complicate the treatment of NOx.
NO2 gas has fairly high solubility and reactivity to water and in aqueous solutions or alkalis as compared with NO, and can be removed by wet scrubbing. On the other hand, gaseous NO is only slightly soluble in water and is not very reactive with typical aqueous solutions. Nitric oxide does react with oxygen as follows:
2NO+O2 = 2NO2
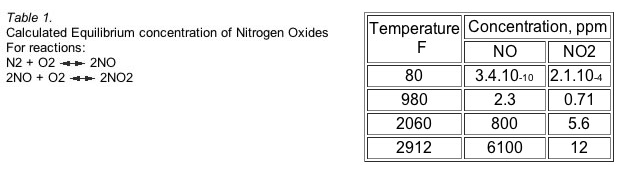
This equation implies the coexistence of NO and NO2. Calculated equilibria indicates that the stability of NO2 decreases with increasing temperature. Nevertheless, from an equilibrium standpoint, the absolute concentration of NO2 increases with temperature while the ratio of its concentration to that of NO decreases with increasing temperature. The equilibrium concentration of NO varies with temperature; it is negligible below 1000 F but quite significant above 2000 F. (Table 1)
 The oxidation of NO is concentration dependent to a marked degree as illustrated in Table 2, which shows the time required for half the NO present in air at various concentrations to be oxidized to NO at ambient temperature. This reaction proceeds more rapidly at a lower temperature than at raised temperatures.
The oxidation of NO is concentration dependent to a marked degree as illustrated in Table 2, which shows the time required for half the NO present in air at various concentrations to be oxidized to NO at ambient temperature. This reaction proceeds more rapidly at a lower temperature than at raised temperatures.
 It may then be concluded that it is impossible to reduce effluent concentration below a few hundred parts per million NOx in absorption equipment of practical dimensions when the entering concentration is in the low percent range. The slow oxidation rate for NO in air can be greatly improved by adding an oxidant such as ozone (O3) or chlorine dioxide (ClO2). The oxidation of NO in the gas phase by ozone or chlorine dioxide occurs much more rapidly than oxidation in the liquid phase because the rate of absorption of NO in the aqueous solution is slow. Ozone is capable of oxidizing NO not only to NO2 but also to N2O5 which rapidly reacts with water or alkaline solutions to form nitric acid or nitrates. Ozone, however, is fairly costly making it usually uneconomical.
It may then be concluded that it is impossible to reduce effluent concentration below a few hundred parts per million NOx in absorption equipment of practical dimensions when the entering concentration is in the low percent range. The slow oxidation rate for NO in air can be greatly improved by adding an oxidant such as ozone (O3) or chlorine dioxide (ClO2). The oxidation of NO in the gas phase by ozone or chlorine dioxide occurs much more rapidly than oxidation in the liquid phase because the rate of absorption of NO in the aqueous solution is slow. Ozone is capable of oxidizing NO not only to NO2 but also to N2O5 which rapidly reacts with water or alkaline solutions to form nitric acid or nitrates. Ozone, however, is fairly costly making it usually uneconomical.
![]()
It has been shown that the use of stoichiometric amounts of ClO2 eliminates approximately 95 percent of the NO in the gas in concentrations of up to at least 24 ppm in less than 2 seconds [4]. Chlorine dioxide is less costly than ozone, but there are inherent difficulties involved in its storage or recovery in terms of equipment maintenance. This is due to its reactive and hazardous nature.
For all practical purposes it is impossible to remove NO gas by wet scrubbing in the situation in which the gas does not contain NO2. It is also known that gaseous NO present in amounts approximately equal to or less than that of gaseous NO2 in a waste gas, when brought into contact with an alkali solution, forms a nitrite and is thereby absorbed as indicated by the formula (2)![]()
If in this case NO2 is present in excess to NO, it reacts with an alkali solution to form nitrate and nitrite and is thereby absorbed as indicated in (3):
![]()
where NO and NO2 are present in equal volumes (NO2:NO mole ratio is 1) reaction (2) will principally proceed, while reaction (3) will become secondary. If the ratio by volume of NO to NO2 is greater than 1 the NO equal in volume to NO2 will react to the nitrite, but the excess NO will essentially remain unchanged. Therefore, the reaction between an alkaline solution and NO/NO2 is optimal at a 1:1 molal ratio of the oxides.
It has been determined that the controlling mechanism of NOx absorption is different according to the relative concentration of NO and NO2. But when the NOx concentration is low, N2O3 (reaction 1) does not form in significant amounts even when the NO:NO2 mole ratio is 1 and the absorption rate is low. The underlying reason is that a high level of NO/NO2 concentration is needed to get the high NOx removal efficiency using an alkaline solution. This means that the complete oxidation of NO to NO2 should be done at low concentrations of NO (less 500 ppm) even when the NO:NO2 ratio of the inlet mixture is 1.
However, when the liquid phase concentration of nitrite and nitrate is relatively high, (Reaction 3) an increase in its concentration causes a decrease in the percent of NO+NO2 removal from the gas, due to the secondary generation of NO that takes place:
![]()
The reaction (4) limits the degree of absorption that takes place using aqueous sodium hydroxide as the scrubbing liquid. Consequently even after the gas phase oxidation of NO to NO2, it is possible to get the new evolution of NO in the liquid phase.
If the gas stream contains NOx and SO2 simultaneously, better results of NOx absorption using sodium hydroxide may be achieved. When this mixture is contacted with aqueous NaOH solution, the SO2 reacts very quickly and forms the sodium sulfite or bisulfite, which can react with NOx. The sodium sulfite exhibits a higher reaction rate with NO2 when compared with sodium hydroxide [3]. Although the reaction mechanisms are not clear, the main reaction that likely occurs when a large excess of sulfite ion is present may be described simply as follows:
![]()
NO, however reacts very poorly even with sulfite solutions. The reaction of NO can be promoted by use of a liquid catalyst. Better results are obtained for the absorption of SO2 and NOx by ammonia solutions containing a soluble catalyst in comparison with aqueous NaOH solution. The overall NO reaction, where the intermediate compounds are ammonium sulfite and bisulfate, may be expressed as:
![]()
It was found that for recovery of 80% of 200ppm NOx the gaseous concentration of SO2 must be more than 1200 ppm [3]. The use of sulfite solution may not be suitable for highly effective removal for large quantities of waste gas particularly when it is oxygen rich resulting in the oxidizing of sulfite to unreactive sulfate. The fact is that a single stage of wet scrubbing cannot provide highly efficient NOx removal generally and especially for NO. For this application, it is preferable to use a first stage for oxidation and second stage for absorption.
An exception to this is the particular NOx Scrubber [6] [7]. This technology utilizes a so-called “surface active media” in a counter current packed tower design generally scrubbing with alkali media. For total NOx concentrations typically >2000 ppmV, NO2:NO mole ratio 2:1 or greater, and O2:NOx mole ratios >5:1 exceptionally high NOx removal (90-99%) can practically be achieved.
Several liquid phase oxidants can be used such as in general hydrogen peroxide potassium permanganate, sodium hypochlorite. The hydrogen peroxide needs care in handling. The potassium permanganate requires the added maintenance to remove the manganese dioxide, a precipitate that forms on the packing. Practically speaking, the most economical of the oxidizing agents is sodium hypochlorite. This usually comes in the form of an alkaline solution to prevent decomposition of sodium hypochlorite to Cl2 and Cl2O and to result in the optimum oxidizing properties. The optimum pH of that scrubbing solution is about 9, where the oxidizing properties of NaOCl are the best. This pH value is where reaction NaOCl NaClO is close to equilibrium and the concentration of NaClO (sodium isohypochlorite) which has the tendency to release the active oxygen is maximum. The optimal pH increases with increasing gas contact time [9]. The oxidizing reaction of NO by sodium isohypochlorite is as follows:
The liquid phase utilized in absorption towers can consist of various chemicals. In this case, alkaline solutions, sodium bisulfite sodium hydrosulfide are used in the scrubbing solutions [10]. For example, when sodium hydrosulfide is used, the NOx reaction may be as follows:
![]()
For oxidizing towers the normal engineering design approach for absorption, based on specific mass-transfer and reaction rate data, is not valid. Accordingly, a large mass-transfer surface is usually required.
For the absorption tower it is suggested to determine the relative effects of mass-transfer and chemical reaction for the absorption of NO-NO2 mixture.
![]()
Where Kga is the overall absorption coefficient kga is the gas-phase mass transfer coefficient and kla is the liquid-phase mass transfer coefficient. The factor is the coefficient which represents the effect of chemical reaction and H is Henry’s law coefficient. The mass transfer coefficient for NO + NO2 were calculated by determining the coefficients for CO2 in water (sparingly soluble gas, liquid phase resistance rate limiting) and SO2 in water (highly soluble gas, resistance of gas and liquid phases comparable) and correcting for the differences in diffusion rates, viscosity’s and densities of CO2, SO2 and NO + NO2. It was concluded that the boundary of chemical interaction between reacting components move toward the liquid surface with increasing liquid flow rate and that the rate is influenced by both diffusion of the active component in the gas and diffusion of the active component as well as the reaction product in the liquid.
A practical and economical design for the wet scrubbing of NOx can therefore be arrived at. However, given the somewhat unusual design factors involved relative to more straight forward absorptive mass transfer chemical systems specific knowledge of the principles involved along with availability of empirical data is critical to determining an effective design.
